SUPPLEMENTARY MATERIAL
Non-pollen palynomorphs from 78 surface sediment samples reveal spatial distribution of phytoplankton in Latvian lakes and ponds
Autori Nimia,b,c,d
a Department of Geography, University of Latvia, Jelgavas iela 1, Riga, LV-1004, Latvia
b Department of Geology, Tallinn University of Technology, Ehitajate tee 5, 19086 Tallinn, Estonia
c Institute of Latvian History, University of Latvia, Kalpaka bulvāris 4, Riga, LV-1050, Latvia
d Lake and Peatland Research Centre, Puikule, Aloja, LV-4063, Latvia
Received 8 January 2023, accepted 10 March 2023, available online
Corresponding author, e-maili aadress
Abstract. The aim of this study is to characterize the current spatial distribution of three main phyla of phytoplankton (Cyanobacteria, Charophyta and Chlorophyta) from 77 Latvian lakes and ponds analysed from modern surface sediment samples through a non-pollen palynomorph approach. The Pearson cross-correlation and the principal component analysis were applied to test the potential correlation of phytoplankton with climate (mean winter and summer temperature), water (pH), environmental (land use – forest, agriculture and urban) and sediment (organic and carbonate matter) variables. The results show the dominance of Chlorophyta in Latvian lakes and ponds. Cyanobacteria were dominant in sites closer to human-populated and recreation centres, including urban and agricultural land-use areas. In more turbid and polluted environments, Chlorophyta thrive today. Charophyta dominated in forested areas. Although Chlorophyta dominate in present-day waterbodies, the rather high relative proportion of Cyanobacteria draws attention to a potential threat. As the cross-correlation results indicate a negative correlation between Cyanobacteria and mean winter temperatures, in warmer climates Cyanobacteria can overtake other phytoplankton. The results of this study can be further used in lake and pond management.
Keywords: non-pollen palynomorphs, algae, gyttja, eutrophication, land use.
INTRODUCTION
Lakes are critical resources for water supplies and food security, as well as highly valued destinations for recreation and tourism. Extensive climate warming over the last few decades has affected lake water temperatures (O’Reilly et al. 2015). A striking consequence of climate change for aquatic ecosystems is that many are experiencing shorter periods of ice cover, as well as earlier and longer summer stratified seasons, which often result in a cascade of ecological and environmental consequence, such as warmer summer water temperatures, alterations in lake mixing and water levels, declines in dissolved oxygen, increased likelihood of cyanobacterial algal blooms, and the loss of habitat for native cold-water organisms (Smol 2008; Woolway et al. 2022).
Establishing the susceptibility and trophic changes in lake ecosystems is an important management issue on a local and regional scale. In this context, a palaeolimnological approach that employs the microfossil record of algae has been considered to be one of the most influential approaches for determining the ecological status of lakes (EC 2000). Lacustrine sedimentary environment is ideal for studying the status of a waterbody. Under limited oxygen conditions, phytoplankton survives for hundreds to thousands of years. There are more than 2300 lakes larger than 1 ha and even more than 10 000 lakes smaller than 1 ha, comprising in total 1.6% of the territory of Latvia (Apsīte and Kļaviņš 2023). Hence, monitoring the current status of lakes is a crucial aspect for maintaining water quality and resulting ecosystem services. In current Latvian legislation, lakes smaller than 50 ha are not distinguished as separate waterbodies. Only if future monitoring data show that this is necessary to achieve environmental quality objectives, this decision will be reviewed. Nevertheless, the majority of small lakes and ponds are not currently monitored in Latvia and there is a lack of information on algal composition.
Considering the average sedimentation rate of 0.5 cm/year (since 1950 CE) in Latvian lakes, the topmost cm comprises approximately the last five years (Stivrins et al. 2021). Hence, the topmost sediment represents modern phytoplankton data that has not been biased by extreme blooming years of algae, but contains smoothed information. In this study, I focus on phytoplankton that has been identified through the non-pollen palynomorphs analysis. This means that acid-resistant freshwater organic-walled algal palynomorphs (hereinafter phytoplankton) have been identified from pollen slides (Stivrins et al. 2022). Phytoplankton comprises a diverse number of phyla, but here diatoms are excluded from Chlorophyta, as they do not survive chemical treatment and are a subject of independent analysis.
The aim of this study is to characterize the current spatial distribution of three main phyla of phytoplankton (Cyanobacteria, Charophyta and Chlorophyta) from Latvian lakes and ponds, analysed from 78 modern surface sediment samples through a non-pollen palynomorph approach. To see possible influencing and driving factors for the presence of phytoplankton in lakes and ponds throughout the territory of Latvia, I use statistical methods to test the relation of phytoplankton to climate and environmental variables. Before proceeding further, it is important to underline that the raw data used in the current study has been previously published and is available to everyone (Stivrins et al. 2022).
STUDY AREA
The study area is Latvia, located in north-eastern Europe within the hemi-boreal vegetation zone. The study sites were natural lakes and human-made waterbodies (ponds, dams and quarries; Fig. 1). In total, 78 surface samples were obtained from 77 waterbodies covering the entire territory of Latvia, and the size of the sampled sites varied from 0.33 to 8070 ha, located along different climate, environmental and geographical gradients. The average annual air temperature in Latvia was +6.8 °C for the climatic normal 1991–2020 and the average annual precipitation 679 mm, according to the Latvian Environment, Geology, and Meteorology Centre data.
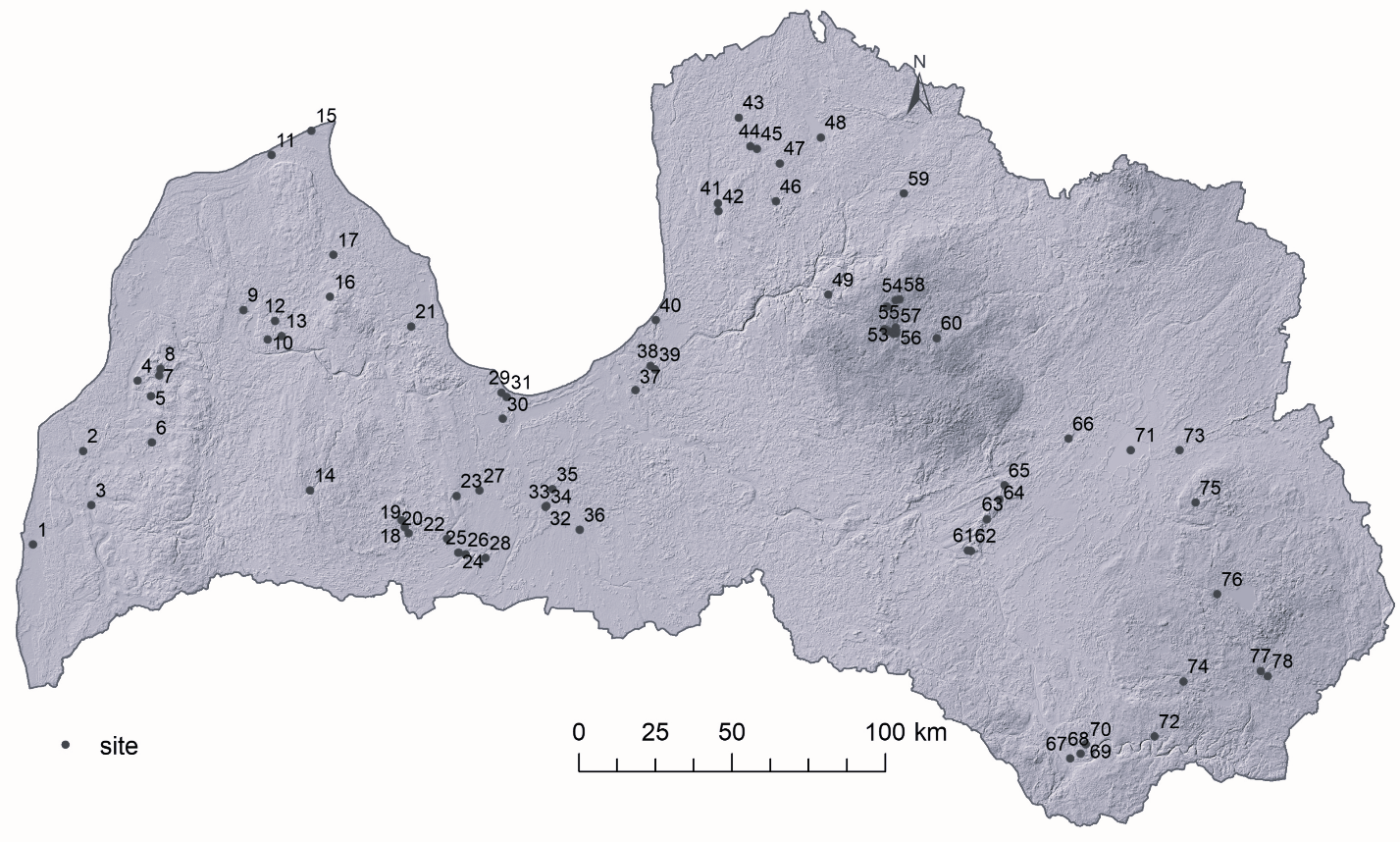
Fig. 1. Location of lakes and ponds in Latvia used in the current article. 1 – Lake Liepājas (3715 ha), 2 – Avotu quarry (1.3 ha), 3 – Lake Durbes (670 ha), 4 – Alsungas dam (7.8 ha), 5 – Lake Zvirgzdu (74.7 ha), 6 – Lake Ķikuru (21.6 ha), 7 – Lake Pinku (29 ha), 8 – Pond Cūku (1.2 ha), 9 – Lake Usmas (3469.2 ha), 10 – Lake Sūnezers (1.4 ha), 11 – Lake Lielais Pēterezers (2.9 ha), 12 – Lake Rūmiķis (1.5 ha), 13 – Lake Vēžezers (7 ha), 14 – Lake Saldus (22 ha), 15 – Vaides dam (5.4 ha), 16 – Lake Talsu (3.6 ha), 17 – Lake Sasmakas (252 ha), 18 – Lake Lielais Vipēdes (20.1 ha), 19 – Lake Sesavas (17 ha), 20 – Lake Spārnu (14 ha), 21 – Lake Vaskaris (22.1 ha), 22 – Lake Apguldes (38.7 ha), 23 – Lake Gauratas (13.8 ha), 24 – Pond Zoltners I (0.5 ha), 25 – Pond Zoltners II (0.2 ha), 26 – Indrānu watershed (24 ha), 27 – Pond Rengas (0.4 ha), 28 – Pūtēļu quarry (7.3 ha), 29 – Lake Melnezers (10.3 ha), 30 – Lake Liliju (2.6 ha), 31 – Lake Slokas (250 ha), 32 – Jelgavas clay quarry II (3.4 ha), 33 – Jelgavas clay quarry I (1 ha), 34 – Jelgavas clay quarry III (3.2 ha), 35 – Lake (quarry) Ozolnieku (18 ha), 36 – Pond (quarry) Staļģenes (1.8 ha), 37 – Lake Velnezers (3.5 ha), 38 – Lake Mazais Baltezers (198.7 ha), 39 – Lake Sekšu (11.7 ha), 40 – Lake Lilastes (183.6 ha), 41 – Lake Limbažu Dūņezers (135.6 ha), 42 – Lake Limbažu Lielezers (256.4 ha), 43 – Lake Ungurpils (22.4 ha), 44 – Pond Grančas (0.4 ha), 45 – Lake Purezers (10 ha), 46 – Lake Augstrozes Lielezers (400 ha), 47 – Lake Mazais Ozolmuižas (17 ha), 48 – Lake Burtnieku (4006 ha), 49 – Lake Āraišu (32.6 ha), 50 – Lake Bānūzis (42.6 ha), 51 – Lake Gulbenes (30.3 ha), 52 – Lake Ilzes (42.4 ha), 53 – Lake Rijas (6 ha), 54 – Lake Asmenis (4.8 ha), 55 – Lake Dabaru (17.8 ha), 56 – Lake Brenkūzis (11.8 ha), 57 – Lake Stupēnu (37.5 ha), 58 – Lake Kaupēnu (11.3 ha), 59 – Lake Trikātas (13 ha), 60 – Lake Bricu (16 ha), 61 – Lake Laukezers (50.4 ha), 62 – Lake Ilzenieku (26.8 ha), 63 – Lake Spiļvu (5.6 ha), 64 – Lake Baltezers (45 ha), 65 – Lake Driksnas (40.5 ha), 66 – Lake Kapu (5.4 ha), 67 – Pond Esplanādes (1.5 ha), 68 – Lake Gubiščes (18.5 ha), 69 – Lake Lielais Stropu (417.9 ha), 70 – Lake Mazais Stropu (15.3 ha), 71 – Lake Lubāns (8070 ha), 72 – Lake Gluhoje (1.7 ha), 73 – Lake Lielais Svētiņu (18.8 ha), 74 – Lake Čertoks (1.9 ha), 75 – Lake Puderovas (9.7 ha), 76 – Lake Zosnas (Rāznas) (156.5 ha), 77 – Pond Dagdas (0.33 ha), 78 – Lake Dagdas (484.1 ha).
MATERIAL AND METHODS
Data on non-pollen palynomorphs, climate and environment are from the project ʽEstablishing training data set of pollen and non-pollen palynomorphs for Latvia – a fundamental ground for climate, landscape, vegetation and water quality reconstructions and modellingʼ (No. LZP-2020/2-0060) and are available in Stivrins et al. (2022). Although the data have been published and discussion presented in the framework of the training-set evaluation of taxa at the species level, the results have not been properly discussed at the phylum level – spatial distribution patterns and the significance for the status of present-day waterbodies. The data set comprises a vast amount of information, and for the current study purposes, I combined species/taxa into three dominant phyla present in the samples – Cyanobacteria, Charophyta and Chlorophyta (Table 1). Then, for the tests, I selected the main climate and environment data that possibly drive the abundance and distribution of phytoplankton in waterbodies.
Table 1. Phytoplankton taxa included in the study
| TAXON NAME [with taxonomic authority] | CLASSIFICATION |
| Anabaena [Bory ex Bornet & Flahault] | Cyanobacteria/Nostocaceae/Anabaena |
| Aphanizomenon [Morren ex Bornet & Flahault] | Cyanobacteria/Aphanizomenonaceae/Aphanizomenon |
| Rivularia [C. Agardh ex Bornet & Flahault] | Cyanobacteria/Rivulariaceae/Rivularia |
| Gloeotrichia [Thuret ex Bornet & Flahault] | Cyanobacteria/Gloeotrichiaceae/Gloeotrichia |
| Gloeotrichia pisum [Thuret ex Bornet & Flahault] | Cyanobacteria/Gloeotrichiaceae/Gloeotrichia |
| Gloeotrichia natans [Rabenhorst ex Bornet & Flahault] | Cyanobacteria/Gloeotrichiaceae/Gloeotrichia |
| Oscillatoriaceae [Engler] | Cyanobacteria/Oscillatoriaceae |
| Spirulina [(Lagerheim) Kirchner] | Cyanobacteria/Spirulinaceae/Spirulina |
| Spirulina [Turpin ex Gomont] | Cyanobacteria/Spirulinaceae/Spirulina |
| Microcystis [Lemmermann] | Cyanobacteria/Microcystaceae/Microcystis |
| Botryococcus braunii [Kützing] | Chlorophyta/Botryococcaceae/Botryococcus |
| Botryococcus neglectus [(West & G. S. West) J.Komárek & P.Marvan] | Chlorophyta/Botryococcaceae/Botryococcus |
| Botryococcus pila [J. Komárek & P. Marvan] | Chlorophyta/Botryococcaceae/Botryococcus |
| Chlamydomonas [Skuja] | Chlorophyta/Chlamydomonadaceae/Chlamydomonas |
| Hariotina reticulata [P. A. Dangeard] | Chlorophyta/Scenedesmaceae/Hariotina |
|
Hariotina polychorda [(Korshikov) E. Hegewald in E. Hegewald, P. F. M. Coesel & P. Hegewald] |
Chlorophyta/Scenedesmaceae/Hariotina |
| Pediastrum boryanum var. boryanum[Meyen] | Chlorophyta/Hydrodictyaceae/Pediastrum |
| Pediastrum duplex var. regulosum [Raciborski] | Chlorophyta/Hydrodictyaceae/Pediastrum |
| Pseudopediastrum boryanum var. longicorne [(Reinsch) Tsarenko] | Chlorophyta/Hydrodictyaceae/Pediastrum |
| Pediastrum boryanum var. brevicorne [A. Braun] | Chlorophyta/Hydrodictyaceae/Pediastrum |
| Pediastrum orientale [(Skuja) V. Jankovská & J. Komárek] | Chlorophyta/Hydrodictyaceae/Pediastrum |
| Pseudopediastrum integrum [(Nägeli) M. Jena & C. Bock in Jena & al.] | Chlorophyta/Hydrodictyaceae/Pseudopediastrum |
| Pediastrum boryanum var. pseudoglabrum [Parra Barrientos] | Chlorophyta/Hydrodictyaceae/Pediastrum |
| Pediastrum boryanum var. cornutum [(Raciborski) Sulek in Fott] | Chlorophyta/Hydrodictyaceae/Pediastrum |
| Pseudopediastrum forcipatum [(Corda) Lenarczyk in Lenarczyk & al.] | Chlorophyta/Hydrodictyaceae/Pseudopediastrum |
| Stauridium tetras [(Ehrenberg) E. Hegewald in Buchheim & al.] | Chlorophyta/Hydrodictyaceae/Stauridium |
| Monactinus simplex var. echinulatum [(Wittrock) Pérez, Maidana & Comas] | Chlorophyta/Hydrodictyaceae/Monactinus |
| Pediastrum simplex var. pseudoglabrum [Parra] | Chlorophyta/Hydrodictyaceae/Pediastrum |
| Monactinus simplex [(Meyen) Corda] | Chlorophyta/Hydrodictyaceae/Monactinus |
| Pediastrum marvillense [Thérézien & Couté ex Parra Barrientos] | Chlorophyta/Hydrodictyaceae/Pediastrum |
| Pediastrum duplex var. gracile [(A. Braun) Raciborski] | Chlorophyta/Hydrodictyaceae/Pediastrum |
| Pediastrum boryanum var. angulosum [(Ehrenberg) Hansgirg] | Chlorophyta/Hydrodictyaceae/Pediastrum |
| Pediastrum duplex var. rugulosum [Raciborski] | Chlorophyta/Hydrodictyaceae/Pediastrum |
| Pediastrum duplex var. asperum [(A. Braun) Hansgirg] | Chlorophyta/Hydrodictyaceae/Pediastrum |
| Pseudopediastrum perforatum [(Raciborski) Lenarczyk in Lenarczyk & al.] | Chlorophyta/Hydrodictyaceae/Pseudopediastrum |
| Pseudopediastrum subgranulatum [(Raciborski) Lenarczyk in Lenarczyk & al.] | Chlorophyta/Hydrodictyaceae/Pseudopediastrum |
| Pseudopediastrum alternans [(Nygaard) M. Jena & C. Bock in Jena & al.] | Chlorophyta/Hydrodictyaceae/Pseudopediastrum |
| Stauridium privum [(Printz) Hegewald in Buchheim & al.] | Chlorophyta/Hydrodictyaceae/Stauridium |
| Scenedesmus [Meyen] | Chlorophyta/Scenedesmaceae/Scenedesmus |
| Scenedesmus arcuatus var. gracilis [(T. Hortobágyi) F. Hindák] | Chlorophyta/Scenedesmaceae/Scenedesmus |
| Tetradesmus obliquus [(Turpin) M. J. Wynne] | Chlorophyta/Scenedesmaceae/Tetradesmus |
| Tetradesmus lagerheimii var. biseriatus [(Reinhard) Taskin & Alp in Taskin & al.] | Chlorophyta/Scenedesmaceae/Tetradesmus |
| Tetradesmus obliquus [(Turpin) M. J. Wynne] | Chlorophyta/Scenedesmaceae/Tetradesmus |
| Desmodesmus perforatus [(Lemmermann) E. Hegewald] | Chlorophyta/Scenedesmaceae/Desmodesmus |
| Tetradesmus dimorphus [(Turpin) M. J. Wynne] | Chlorophyta/Scenedesmaceae/Tetradesmus |
| Desmodesmus denticulatus [(Lagerheim) S. S. An, T. Friedl & E. Hegewald] | Chlorophyta/Scenedesmaceae/Desmodesmus |
| Scenedesmus obtusus [Meyen] | Chlorophyta/Scenedesmaceae/Scenedesmus |
| Desmodesmus communis [(E. Hegewald) E. Hegewald] | Chlorophyta/Scenedesmaceae/Desmodesmus |
| Desmodesmus opoliensis [(P. G. Richter) E. Hegewald] | Chlorophyta/Scenedesmaceae/Desmodesmus |
| Desmodesmus bicaudatus [(Dedusenko) P. M. Tsarenko] | Chlorophyta/Scenedesmaceae/Desmodesmus |
| Scenedesmus armatus [(Chodat) Chodat] | Chlorophyta/Scenedesmaceae/Scenedesmus |
| Scenedesmus quadricauda var. lefevrii [(Delandre) Dedusenko in Korshikov] | Chlorophyta/Scenedesmaceae/Scenedesmus |
| Desmodesmus perforatus [(Lemmermann) E. Hegewald] | Chlorophyta/Scenedesmaceae/Desmodesmus |
| Desmodesmus pannonicus [(Hortobágyi) E. Hegewald] | Chlorophyta/Scenedesmaceae/Desmodesmus |
| Scenedesmus ellipticus [Corda] | Chlorophyta/Scenedesmaceae/Scenedesmus |
| Lemmermannia triangularis [(Chodat) C. Bock & Krienitz in C. Bock & al.] |
Chlorophyta/Trebouxiophyceae incertae sedis/Lemmermannia |
| Tetrastrum staurogeniiforme [(Schröder) Lemmermann] | Chlorophyta/Scenedesmaceae/Tetrastrum |
| Pleurococcus [Meneghini] | Chlorophyta/Chaetophoraceae/Pleurococcus |
| Planctonema lauterbornii [Schmidle] | Chlorophyta/Oocystaceae/Planctonema |
| Crucigenia fenestrata [(Schmidle) Schmidle] |
Chlorophyta/Trebouxiophyceae incertae sedis/Crucigenia |
| Lemmermannia tetrapedia [(Kirchner) Lemmermann] |
Chlorophyta/Trebouxiophyceae incertae sedis/Lemmermannia |
| Tetraëdron minimum [(A. Braun) Hansgirg] | Chlorophyta/Hydrodictyaceae/Tetraedron |
| Tetraëdron triangulare [Korshikov] | Chlorophyta/Hydrodictyaceae/Tetraedron |
| Tetraëdron trigonum [(Nägeli) Hansgirg] | Chlorophyta/Hydrodictyaceae/Tetraedron |
| Treubaria schmidlei [(Schröder) Fott & Kovácik] | Chlorophyta/Treubariaceae/Treubaria |
| Tetraëdron caudatum [(Corda) Hansgirg] | Chlorophyta/Hydrodictyaceae/Tetraedron |
| Cosmarium baileyi [Wolle] | Charophyta/Desmidiaceae/Cosmarium |
| Cosmarium franzstonii [Taft] | Charophyta/Desmidiaceae/Cosmarium |
| Cosmarium formosulum [Hoff in Nordstedt] | Charophyta/Desmidiaceae/Cosmarium |
| Cosmarium variolatum [P. Lundell] | Charophyta/Desmidiaceae/Cosmarium |
| Cosmarium protractum [(Nägeli) De Bary] | Charophyta/Desmidiaceae/Cosmarium |
| Cosmarium pseudopyramidatum [Willi Krieger & Gerloff] | Charophyta/Desmidiaceae/Cosmarium |
| Cosmarium turpinii [Brébisson] | Charophyta/Desmidiaceae/Cosmarium |
| Euastrum lacustre [(Messikommer) Coesel] | Charophyta/Desmidiaceae/Euastrum |
| Euastrum bidentatum [Nägeli] | Charophyta/Desmidiaceae/Euastrum |
| Euastrum luetkemuelleri [F. Ducellier] | Charophyta/Desmidiaceae/Euastrum |
| Staurastrum [Ralfs ex Ralfs] | Charophyta/Desmidiacae/Staurastrum |
| Staurastrum ophiura var. pentacerum [Wolle] | Charophyta/Desmidiaceae/Staurastrum |
| Staurastrum pingue [Teiling] | Charophyta/Desmidiacae/Staurastrum |
| Staurastrum pingue var. planctonicum [(Teiling) Coesel & Meersters] | Charophyta/Desmidiaceae/Staurastrum |
| Staurastrum tetracerum [Ralfs ex Ralfs] | Charophyta/Desmidiacae/Staurastrum |
| Staurastrum anatinum [Cooke & Wills] | Charophyta/Desmidiacae/Staurastrum |
| Staurastrum punctulatum [Brébisson in Ralfs] | Charophyta/Desmidiacae/Staurastrum |
| Staurastrum gracile [Ralfs ex Ralfs] | Charophyta/Desmidiacae/Staurastrum |
| Staurastrum arachne [Ralfs ex Ralfs] | Charophyta/Desmidiacae/Staurastrum |
| Spirogyra [Link, nom. cons] | Charophyta/Zygnemataceae/Spirogyra |
| Transeauina glyptosperma [(De Bary) Guiry] | Charophyta/Zygnemataceae/Transeauina |
| Mougeotia capucina [C. Agardh] | Charophyta/Zygnemataceae/Mougotia |
| Zygnema [C. Agardh] | Charophyta/Zygnemataceae/Zygnema |
A 3 cm top sediment sample was obtained from the waterbodies using a gravity corer (KC Denmark) through ice in winter 2021 and from a boat in winter, spring-summer 2020–2021. A total of 78 samples were treated for non-pollen palynomorph (NPP) analysis, as explained below. Initial work on smear slides showed that the identification process was challenging, and therefore, certain treatment steps were necessary (Stivrins et al. 2022). Hydrochloric acid (HCl 10%) was added to a 1 cm3 sample to remove carbonates and dissolve the Lycopodium tablet (Stockmarr 1971), after which 5% potassium hydroxide (KOH) was added (water bath 80 °C, 5 min) to react the depolymerized humic acids with their conjugate base pairs to produce soluble organic salts, which were then removed by washing with water. The prepared samples were stored in glycerol. Currently, there is no consensus among NPP scientists on how long or how many remains should be counted. In this case I relied on a constant exotic marker count, i.e., I counted NPPs until 100 Lycopodium spores were reached per sample. NPPs were identified using published literature (e.g., Bellinger and Sigee 2010; Miola 2012; Guiry and Guiry 2021).
| STATION | LONGITUDE / LATITUDE | N NE E SE S SW W NW |
| Vilsandi | 58° 22′ 58″ 21° 48′ 51″ | + + ~ ~ + + ~ + |
| Sõrve | 57° 54′ 49″ 22° 03′ 29″ | ~ ~ + + + + + ~ |
| Kihnu | 58° 05′ 55″ 23° 58′ 12″ | ‒ ‒ ~ ~ + + + ~ |
| Kunda | 59° 31′ 04″ 26° 32′ 43″ | + + ‒ ‒ ‒ ‒ ‒ + |
| Kalbåda | 59° 59′ 08″ 25° 35′ 56ʺ | + + + + + + + + |
Jorg,Y itself combines the total organ flow rate Jorg with large/small pore hydraulic conductance aY and isogravimetric flow Jiso,org.Y, where the latter is a local transvascular oncotic pressure gradient-driven circulation:
Jorg, L = Jiso, org + αL • Jorg, (6)
Jorg, S = −Jiso, org + αS • Jorg. (7)
u = –WS sin q; v = –WS cos q, (8)
where WS is the wind speed (in m/s), and q is the wind direction (compass degrees recalculated into radians). One should bear in mind that the presentation of u and v vector components (see inset in Fig. 1A) applies similarly to both winds and sea currents, but there is a historical controversy regarding the directions in the compass rose system: the wind blows ‘into the compass’, but the sea current ‘flows out of the compass’.
Land cover was characterized using Corine Land Cover 2018 data (EEA 2020) with aggregated thematic land cover categories – forest, agricultural area, and urban area (within a 1 km buffer zone). Information on the climate at each site was derived from the nearest meteorological station (climatic normal for 1991–2020) collected by the Latvian Environment, Geology, and Meteorology Centre. Water properties (pH) were measured from a boat from May to August (Fig. 2) using a WTW multimeter. The relative composition of sediment (organic and carbonate matter, %) was measured through the loss-on-ignition method (Dean 1974).

Fig. 2. A – water property measurements from a boat in Lake Sesavas (site 19 in Fig. 1). B – the loss-on-ignition analysis at the University of Latvia.
In this study, I used the Pearson cross-correlation and the principal component analysis (PCA) to test the potential correlation of phytoplankton with climate (mean winter and summer temperature), water (pH), environment (land use – forest, agriculture and urban) and sediment (organic and carbonate matter) variables. Pearson’s r is the most commonly used parametric correlation coefficient. Prior to the correlation, I used a logarithmic data transformation with the purpose of making data more normally distributed (Hammer et al. 2001). The PCA finds components accounting for as much of the variance in data as possible (Davis 1986; Legendre and Legendre 1998), which were tested using the broken stick method (Jackson 1993) in Past v.4.11 (Hammer et al. 2001).
RESULTS
In total, 88 species/taxa comprise three phyla (Cyanobacteria = 10, Chlorophyta = 55 and Charophyta = 23). Chlorophyta dominated in 65 waterbodies (Fig. 3), Cyanobacteria in ten waterbodies and the lowest dominance was for Charophyta in two sites (Lake Čertoks (site 74 in Fig. 1) and Vaides dam (site 15 in Fig. 1)). Within 17 waterbodies >50 ha, Chlorophyta dominated in 15 sites, while Cyanobacteria prevailed in two lakes. If looking at smaller sites, such as <10 ha, Cyanobacteria dominated in six out of 29 waterbodies. Although only ten sites in total were dominated by Cyanobacteria, the majority of the sites (seven out of ten; 70%) were located in cities and in high human-induced activity zones. Charophyta dominated in forested regions with limited human activities nearby. Meanwhile, Chlorophyta dominated in different regions and settings (Fig. 3).
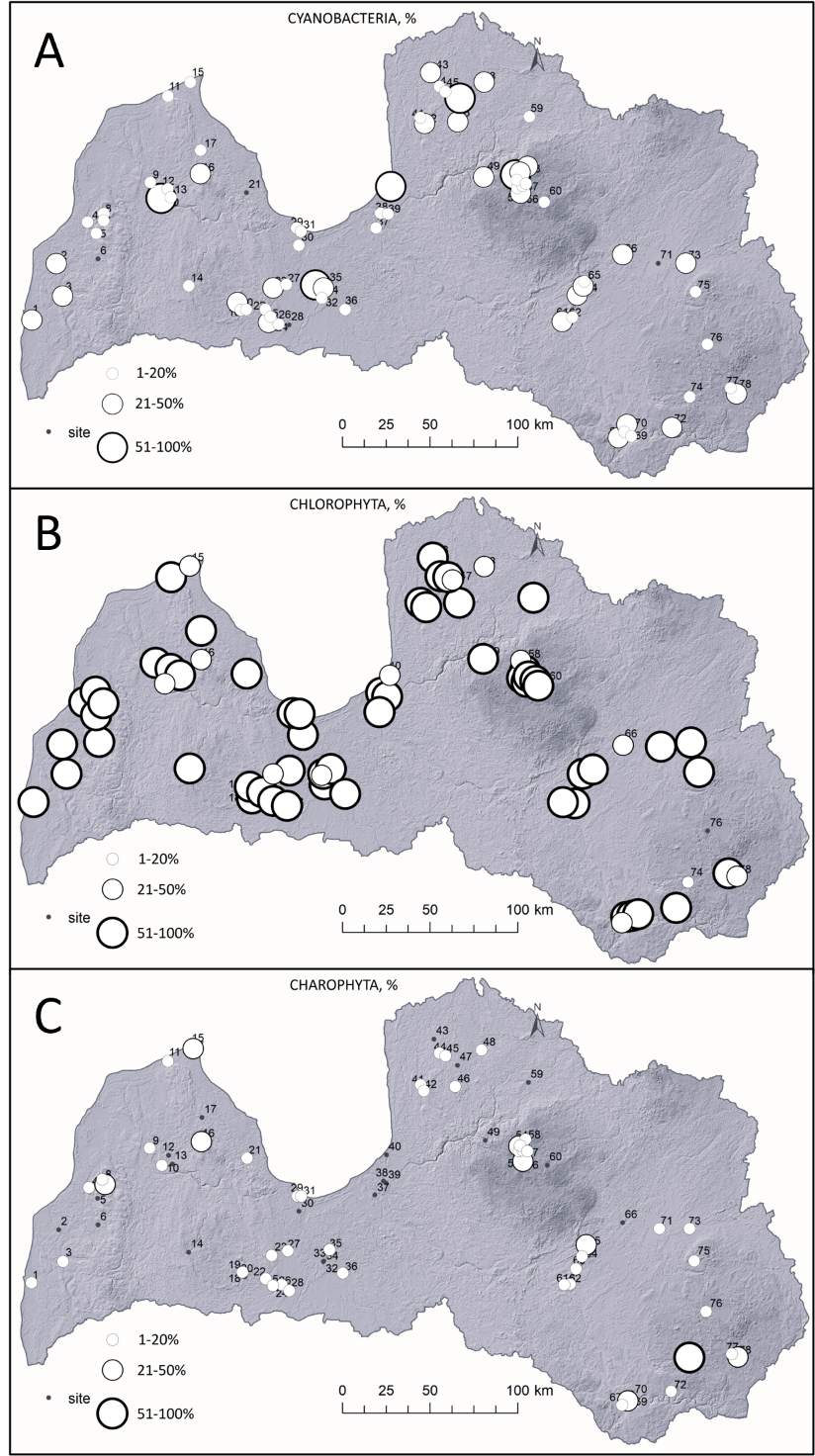
Fig. 3. Spatial distribution and relative coverage of Cyanobacteria (A), Chlorophyta (B) and Charophyta (C) in the lakes and ponds of Latvia. Names of each site are shown in Fig. 1.
The PCA results indicate that Chlorophyta can dominate in forested, agricultural, paludified and urban settings (Fig. 4). Hence, it is a matter of species/taxa composition, which can then be used for characterizing a waterbody’s trophic status more precisely. The PCA results show correlation between Cyanobacteria, urban and agricultural settings. Although a few sites dominated by Cyanobacteria are located in forested regions, these sites are mainly known to be influenced by anthropogenic activities. Charophyta phyla show a correlation between increased organic matter and forested regions.
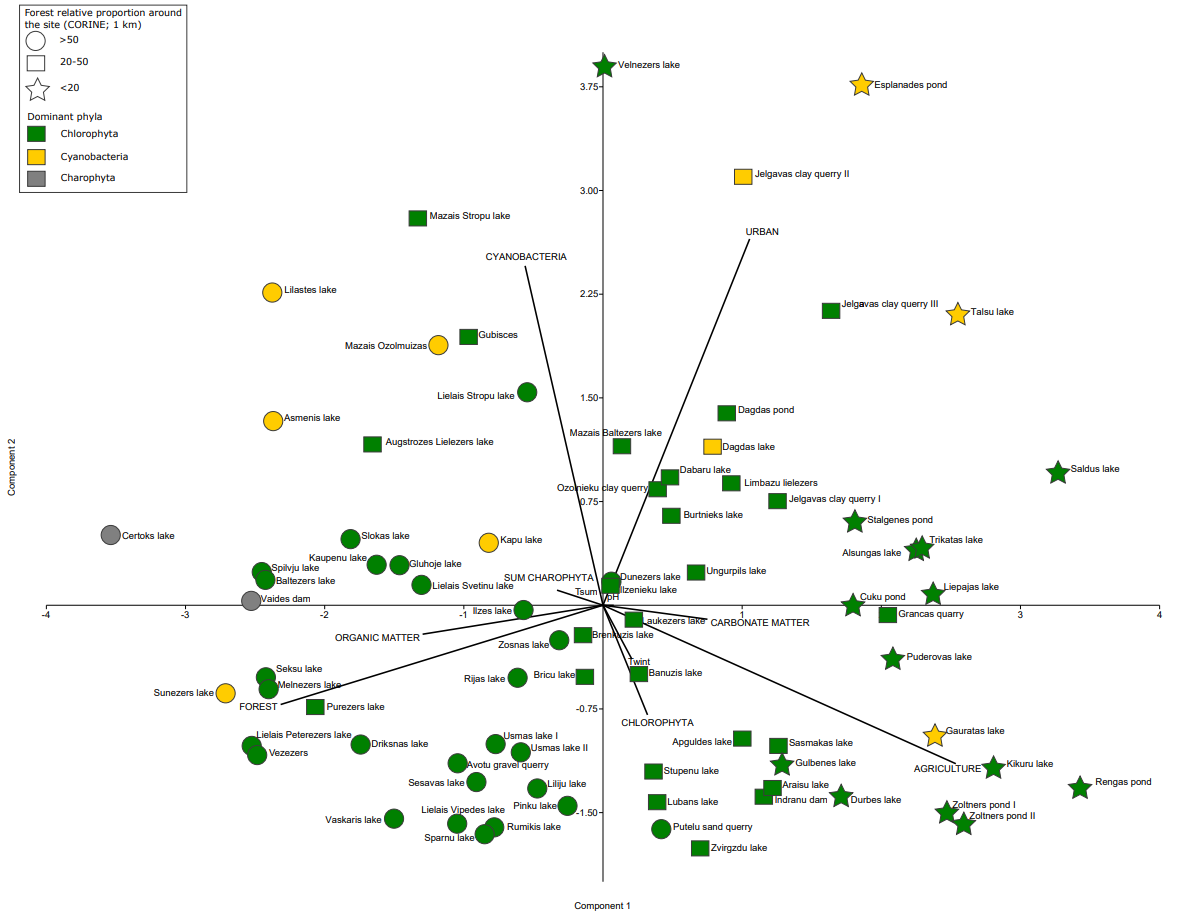
Fig. 4. Principal component analysis of modern phytoplankton in waterbodies and distribution between sites, indicating both the relative share of forest (CORINE) within a 1 km radius around the waterbodies (circle – >50%, square – 20–50% and star – <20% forest cover) and the dominant phytoplankton phyla in a particular site (green – Chlorophyta, yellow – Cyanobacteria, grey – Charophyta), as well as climate (Tsum – mean summer temperature, Twint – mean winter temperature), sediment composition (organic and carbonate matter), water (pH) and land-use (urban, forest, agricultural) variables.
The results of Pearson correlation reveal both significantly positive and negative correlations (Fig. 5), i.e., Cyanobacteria were significantly negatively correlated with carbonate matter content and winter temperature, while a somewhat positive correlation was found between Cyanobacteria, urban and forest variables. Chlorophyta were significantly positively correlated with agriculture and winter temperature. A minor positive correlation was observed between Chlorophyta and carbonate matter content, and a negative correlation with forest and urban factors (Fig. 5). Charophyta did not show significant correlations, but a potential significant correlation seems to be with forest and a negative correlation with agriculture and winter temperature (Fig. 5).
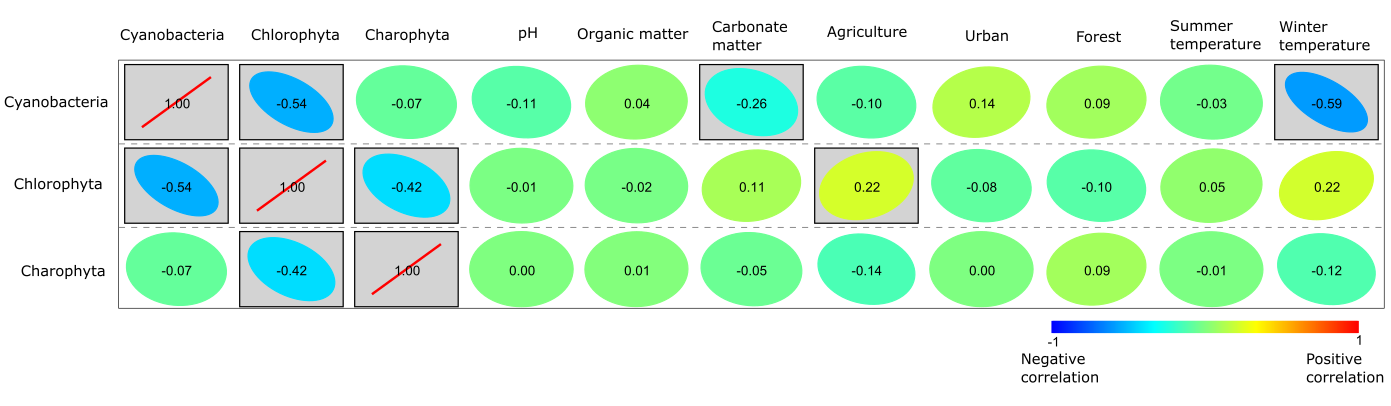
Fig. 5. Pearson correlation between Cyanobacteria, Chlorophyta, Charophyta and the variables of climate (mean summer and winter temperature), environment (water pH, land use – agriculture, urban, forest) and sediment (organic and carbonate matter). Significance p < 0.05 is boxed, colour: from red – significantly positive to blue – significantly negative.
DISCUSSION
Before discussing algae, it is important to underline the heterogeneity of waterbodies in Latvia. Each studied site differs and so do the modern sediments. For instance, in a 40-year-old man-made quarry, new organic sediment has accumulated, indicating the beginning of the waterbody infilling process (Fig. 3). Few natural lakes (mainly coastal) tend to have less organic matter (Fig. 3) than smaller sites in other locations. These differences in ecosystems, influenced by exodynamic processes and the ontogeny of individual waterbodies, regulate the current algal composition.
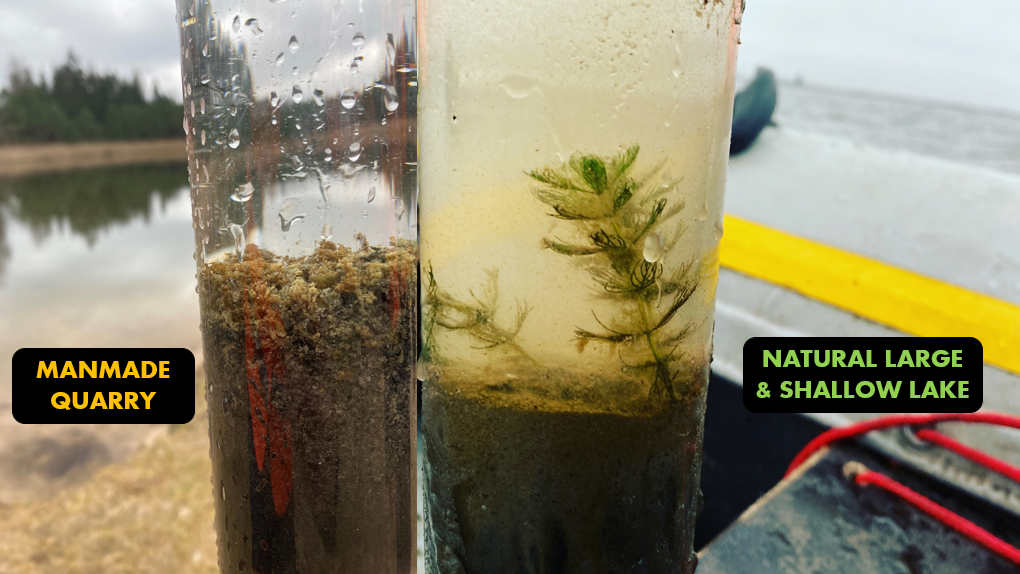
Fig. 6. Topmost sediment obtained from a man-made quarry (Avotu quarry (site 2 in Fig. 1)) and a large shallow natural lake (Lake Liepājas (site 1 in Fig. 1)). Note the difference in the concentration of suspended material within the water column.
Three dominant phyla of algae are present in current waterbodies of Latvia – Chlorophyta, Cyanobacteria and Charophyta (Fig. 3). Algae per se do not imply that the waterbody is in poor conditions, because they are part of living organisms within the lacustrine ecosystem (Hou et al. 2022).
Chlorophyta dominate in Latvian lakes and ponds, and the dominance is a matter of geographical location, land use and other environmental factors. Under higher agricultural pressure, the waterbody is enriched with additional nutrient supply leading to eutrophication, which is supported by the Pearson correlation showing a significantly positive correlation between Chlorophyta, agriculture and winter temperature (Fig. 6). Due to warmer winters, we might expect a decrease in Chlorophyta, but agricultural activities will continue and therefore also the presence and dominance of Chlorophyta in different waterbodies. Chlorophyta comprise a wide range of taxa and, therefore, it is not possible to state the actual health status of a waterbody from the phyla alone. Chlorophyta appear in agricultural, forested, urban and even peatland areas (Fig. 1). While for Cyanobacteria and Charophyta it can be possible to underline the trophic status or current status of the waterbody, it is challenging in the case of Chlorophyta, where taxa and species must be listed in the first place. Algae are influenced by an array of factors, such as climate (precipitation, temperature), brownification (dissolved organic carbon (DOC), dissolved inorganic carbon (DIC)), eutrophication, chemical leakage and also biota (Feuchtmayr et al. 2019; Stivrins et al. 2022).
In small waterbodies located within forested regions, one might expect to see higher Charophyta abundance, as is evident from the results (Figs 3–5, 7). Investigation on Charophyta should be continued because more samples and sites need to be studied to gain higher confidence about their present distribution and environmental requirements in Latvian waterbodies.
There is a significantly negative correlation between Cyanobacteria and mean winter temperature (Fig. 5), indicating that warmer winters favour the dominance of Cyanobacteria, giving them a competitive advantage over other phyla of phytoplankton. Higher winter temperature could affect water temperature, resulting in the lower water level and increased lake residence time, leading to prolonged periods of thermal stratification of the water column (De Senerpont Domis et al. 2013). The significantly negative correlation between Cyanobacteria and carbonate matter in the Pearson correlation, and the close relationship between carbonate matter and agriculture in the PCA possibly suggest that turbulent water and increased nutrient supply disrupts favourable living conditions for Cyanobacteria.
Although Cyanobacteria can be found naturally in waterbodies when certain conditions exist, such as warm water rich in nutrients, Cyanobacteria can rapidly form harmful algal blooms. According to the results of this study, Cyanobacteria can occur more frequently in waterbodies of less than 50 ha that are located in urban or anthropogenic stress settings (Fig. 7). The positive statistical correlation between Cyanobacteria and urban land use underlines the anthropogenic drive for Cyanobacteria (Fig. 5). The surroundings of Lake Lilaste (site 40 in Fig. 1) are >50% pine forest, but since wastewater from the residential area is discharged into the lake, it is polluted, leading to the dominance of Cyanobacteria (Fig. 3). Similarly, Pond Esplanades (site 67 in Fig. 1) located in the centre of the 2nd largest city in Latvia – Daugavpils – has a rather high anthropogenic stress setting, supporting the dominance of Cyanobacteria. Lake Gauratas (site 23 in Figs 1, 3) – an official swimming site for the city of Dobele and characterized by agricultural land use – has an increased presence of Cyanobacteria. Sites dominated by Cyanobacteria should be addressed in the first place, because within the identified taxa were Anabaena and Aphanizomenon, which are able to develop blooms and produce toxic metabolites dangerous for humans and living organisms, including hepatotoxins, neurotoxins and cytotoxins (Österholm et al. 2020; Osburn et al. 2022).
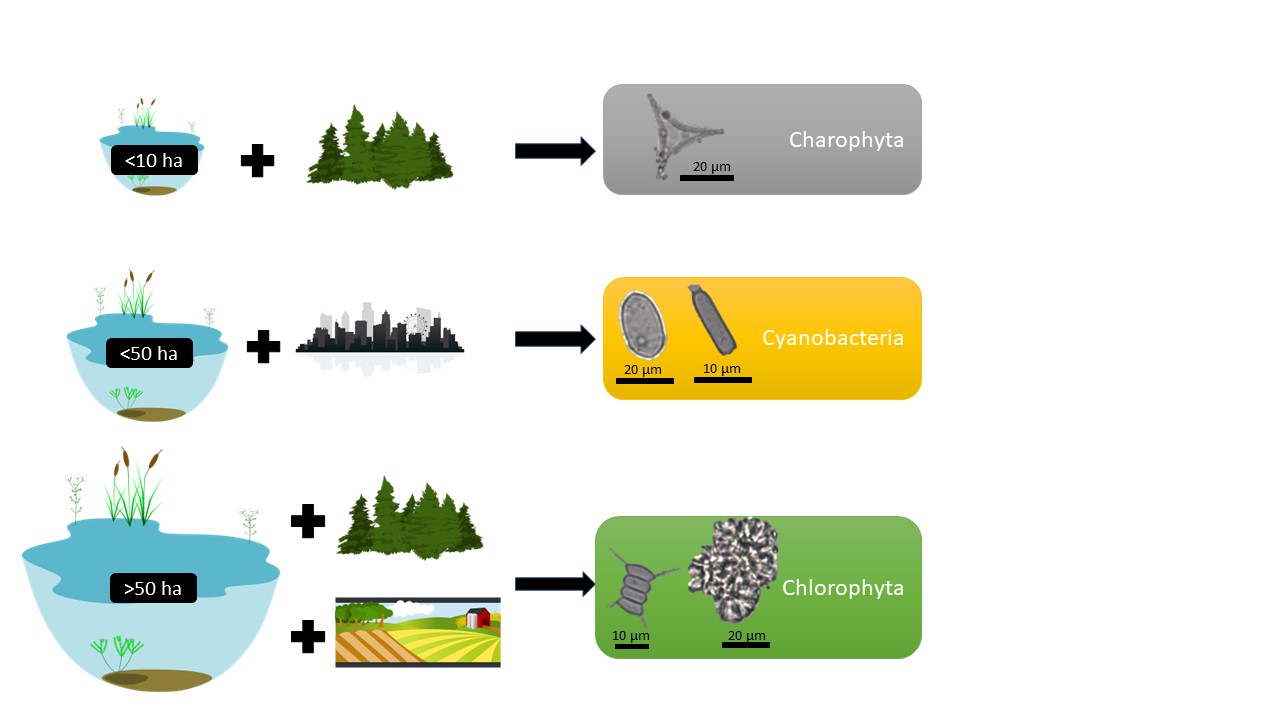
Fig. 7. Conceptual model of phyla dominance in waterbodies according to the findings of this study. Starting from the top: waterbodies <10 ha in natural and forested areas favour the living conditions for Charophyta; waterbodies <50 ha influenced by human activities and located in urban context favour the living conditions for Cyanobacteria; waterbodies >50 ha in forested or agricultural land-use setting favour the living conditions for Chlorophyta.
Waterbody management authorities and upcoming projects are welcome to use these results providing baseline information on the composition and presence of algae. From an applied point of view, these results should be considered together with information on water chemistry, as it is necessary to identify the possible reasons for algal presence/dominance to ensure that the right measures are taken for water quality improvement (e.g., closing wastewater inflow, cleaning a waterbody of sediment, etc.). Even without specific water chemical data based on the results of the current study and visiting the sites during the fieldwork, I see a need to proceed with implementing water management for the following sites: Lake Talsu (site 16 in Fig. 1), Lake Gauratas (site 23 in Fig. 1), Pond Zoltners I and II (sites 24 and 25 in Fig. 1), Lake Lilastes (site 40 in Fig. 1), and Lake Trikātas (site 59 in Fig. 1). These sites are influenced by human activities and therefore visible algae blooming often occurs there. As algal composition differs from site to site, there is no single suggestion on how to proceed, but some action must be taken to improve the health of Latvian waterbodies.
CONCLUSIONS
The current study presents an overview of the distribution of the main phytoplankton phyla – Cyanobacteria, Chlorophyta and Charophyta – in Latvian lakes and ponds. Using previously published data sets, I investigated general patterns in the spatial distribution of phytoplankton in relation to available climate and environmental variables. By means of the principal component analysis and the Pearson cross-correlation tests, it was possible to unravel current possible driving factors for phytoplankton spatial abundance. As expected, the dominant phylum was Chlorophyta (dominant in 65 waterbodies), followed by Cyanobacteria (dominant in 10 sites) and least but not last Charophyta (dominant in two sites). Not only human impact (through agricultural and urban activities), but also climate (increase in mean winter temperature) influences the present-day phytoplankton composition in Latvian lakes and ponds. This study shows that there are numerous sites requiring attention for waterbody management and raising awareness for the current climate impact behind Cyanobacteria dominance in some of the Latvian waterbodies.
Acknowledgements. In Estonia, the project was funded by the Estonian Research Council through PRG323. Additional funding was provided by the performance-based national basic research funding of the University of Latvia under the programme ʽClimate change and sustainable use of natural resourcesʼ (Y5-AZ03_ZF-N-110). The original study data were obtained from the Latvian Council of Science funded project No. LZP-2020/2-0060. Lyudmila Shumilovskikh and an anonymous reviewer are thanked for their valuable comments. The publication costs of this article were covered by the Estonian Academy of Sciences. Special thanks to my wife Karīna and daughter Elīza for their kind support throughout these years.
REFERENCES
Apsīte, E. and Kļaviņš, M. 2023. Iekšējie virszemes ūdeņi Latvijā (Inland surface waters in Latvia). Nacionālā enciklopēdija (National Encyclopaedia).
https://enciklopedija.lv/skirklis/26188-iekšējie-virszemes-ūdeņi-Latvijā
Guiry, M. D. and Guiry, G. M. 2021. AlgaeBase. World-wide electronic publication. National University of Ireland, Galway.
https://www.algaebase.org (accessed 2021-11-22).
Bellinger, E. G. and Sigee, D. C. 2010. Freshwater Algae, Identification and Use as Bioindicators. Antony Rowe, Chippenham.
https://doi.org/10.1002/9780470689554
Davis, J. C. 1986. Statistics and Data Analysis in Geology. John Wiley & Sons, Hoboken, NJ.
De Senerpont Domis, L. N., Elser, J. J., Gsell, A. S., Huszar, V. L. M., Ibelings, B. W., Jeppesen, E. et al. 2013. Plankton dynamics under different climatic conditions in space and time. Freshwater Biology, 58, 463–482.
https://doi.org/10.1111/fwb.12053
Dean, W. E. 1974. Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: comparison with other methods. Journal of Sedimentary Petrology, 44, 242–248.
https://doi.org/10.1306/74D729D2-2B21-11D7-8648000102C1865D
EC (European Community). 2000. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Official Journal of the European Communities, L327, 1–17.
EEA (European Environmental Agency). 2020. Corine Land Cover 2018 Data. Copernicus Land Monitoring Service.
https://land.copernicus.eu/pan-european/corine-land-cover/clc2018 (accessed 2022-06-04)
Feuchtmayr, H., Pottinger, T. G., Moore, A., De Ville, M. M., Caillouet, L., Carter, H. T. et al. 2019. Effects of brownification and warming on algal blooms, metabolism and higher trophic levels in productive shallow lake mesocosms. Science of The Total Environment, 678, 227–238.
https://doi.org/10.1016/j.scitotenv.2019.04.105
Hammer, Ø., Harper, D. A. T. and Ryan, P. D. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4.
Hou, X., Feng, L., Dai, Y., Hu, C., Gibson, L., Tang, J. et al. 2022. Global mapping reveals increase in lacustrine algal blooms over the past decade. Nature Geoscience, 15, 130–134.
https://doi.org/10.1038/s41561-021-00887-x
Jackson, D. A. 1993. Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology, 74, 2204–2214.
https://doi.org/10.2307/1939574
Legendre, P. and Legendre, L. 1998. Numerical Ecology. 2nd ed. Elsevier, Amsterdam.
Miola, A. 2012. Tools for Non-Pollen Palynomorphs (NPPs) analysis: a list of Quaternary NPP types and reference literature in English language (1972–2011). Review of Palaeobotany and Palynology, 186, 142–161.
https://doi.org/10.1016/j.revpalbo.2012.06.010
O’Reilly, C. M., Sharma, S., Gray, D. K., Hampton, S. E., Read, J. S., Rowley, R. J. et al. 2015. Rapid and highly variable warming of lake surface water around the globe. Geophysical Research Letters, 42, 10773–10781.
https://doi.org/10.1002/2015GL066235
Osburn, F. S., Wagner, N. D., Taylor, R. B., Chambliss, C. K., Brooks, B. W. and Scott, J. T. 2022. The effects of salinity and N : P on N-rich toxins by both an N-fixing and non-N-fixing cyanobacteria. Limnology and Oceanography Letters, 8, 162–172.
https://doi.org/10.1002/lol2.10234
Österholm, J., Popin, R. V., Fewer, D. P. and Sivonen, K. 2020. Phylogenomic analysis of secondary metabolism in the toxic Cyanobacterial genera Anabaena, Dolichospermum and Aphanizomenon. Toxins, 12, 248.
https://doi.org/10.3390/toxins12040248
Smol, J. P. 2008. Pollution of Lakes and Rivers: a Paleoenvironmental Perspective. 2nd ed. Blackwell Publishing, Oxford.
Stivrins, N., Briede, A., Steinberga, D., Jasiunas, N., Jeskins, J., Kalnina, L. et al. 2021. Natural and human-transformed vegetation and landscape reflected by modern pollen data in the boreonemoral zone of northeastern Europe. Forests, 12, 1166.
https://doi.org/10.3390/f12091166
Stivrins, N., Trasune, L., Jasiunas, N., Kalnina, L., Briede, A., Maksims, A. et al. 2022. Indicative value and training set of freshwater organic-walled algal palynomorphs (non-pollen palynomorphs). Quaternary Science Reviews, 282, 107450.
https://doi.org/10.1016/j.quascirev.2022.107450
Stockmarr, J. 1971. Tablets with spores used in absolute pollen analysis. Pollen et Spores, 13, 615–621.
Woolway, R. I., Sharma, S. and Smol, J. P. 2022. Lakes in hot water: the impacts of a changing climate on aquatic ecosystems. BioScience, 72, 1050–1061.
https://doi.org/10.1093/biosci/biac052



