TECHNOGENIC MINERALS IN THE WASTE ROCK HEAPS OF
ESTONIAN OIL SHALE MINES
AND THEIR USE TO PREDICT THE ENVIRONMENTAL IMPACT OF THE WASTE
Department of Chemical Engineering and Technology
Royal Insitute of Technology
Stockholm, S-10044 Sweden
&
Institute of Geology
University of Tartu
46 Vanemuise, Tartu 51014, Estonia
The newly formed minerals inside the waste rock heaps of
Estonian oil shale mines were studied using X-ray diffraction analysis and
scanning electron microscopy. In the unburnt heaps, the only detectable new
solid phases were ferric oxyhydroxide and gypsum. In the burnt heaps, however,
a large number of technogenic minerals were found, including lime, periclase,
portlandite, brussite, calcite, aragonite, leucite, diopside, gehlenite,
wollastonite, anhydrite, ettringite, hematite, tobermorite, larnite and
spurrite.
The
reasons for spontaneous combustion include heap size and shape, but importantly
also the heterogeneities, especially gravitational fractionation of the rocks
during the disposal. The areas where the shale remains not fully oxidised were
found, characterised by the presence of oil in the surface layer and layers of
amorphous carbon on the waste rock lumps. The negative environmental impacts of
the burnt heaps, including leachate with high alkalinity and sulfate content,
as well as oil plumes, may become evident after decades after burning only,
because the temperature inside the heaps decreases very slowly.
Introduction
Estonian oil shale mines produce kukersite for
processing and power generation. In all of these operations, large amounts of
solid residues are produced. About a third of the rock mass excavated by
underground mining is the waste rock, separated during enrichment and disposed
in heaps with the height of several tens of meters. The mining peaked in early
1980’s when about 7-8 Mt waste rock was disposed each year. By 1998, about 200
Mt of the waste rock was disposed in heaps.
In order to understand the
impact of the waste rock on the environment, the study of the solid phases of
the waste is needed. These solid phases are here named the technogenic
minerals, although the process of generation of many of them is analogous to
the natural geological processes, such as thermal metamorphism and chemical
sedimentation from oversaturated water solutions. The technogenic minerals and
their changes in time determine the quality of the leachate leaking from the
waste, with the possibility of being directly responsible for water pollution.
The mineral composition of the
kukersite waste has not been a subject of an extensive research. The reason is
that the waste rock undergoes small chemical changes only, mainly because of
the oxidation of pyrite, without causing serious environmental problems. So,
the composition of the waste rock is approximated to the unchanged shale and
limestone. However, some of the waste rock heaps have suffered spontaneous
combustion, totally changing the composition of solids.
The aim of this study was to
document the solid phase composition of the altered waste rock, especially that
of the burnt heaps, together with the geochemical analysis of the processes of
change. The study assists to work out the guidelines on the hazardousness and
water pollution potential of the waste rock.
Materials and methods
The samples of the waste rock were taken and
analysed by means of X-ray diffraction during 1988-1998. The XRD data were
collected scanning unoriented powdered samples with DRON-0.5, DRON-3M and
Siemens diffraction systems using FeK and CuK
and CuK radiation. The
mineralogy of the unchanged oil shale was analysed quantitatively using the
methods described by Utsal in 1984 [1]. The sample of the black surface layer
from the Kukruse burnt waste rock heap was also analysed using PHILIPS XL30
scanning electron microscope, equipped with the energy dispersive spectrometry
system.
radiation. The
mineralogy of the unchanged oil shale was analysed quantitatively using the
methods described by Utsal in 1984 [1]. The sample of the black surface layer
from the Kukruse burnt waste rock heap was also analysed using PHILIPS XL30
scanning electron microscope, equipped with the energy dispersive spectrometry
system.
The computer code PHREEQC [2] and the Wateq4 database [3] were used to
characterise some geochemical interactions in the water phase.
Mineralogy of kukersite
Kukersite is at present the only economically
used oil shale in Estonia and belongs to the general category of carbonate-rich
shales. The organic matter is yellowish-brown to dark-brown kerogen containing
algal remains with the size between 10-40mm [4]. Values of the kerogen
content of productive seams are ranging between 30-60 %.
The estimations of the mineral composition
calculated for 48 samples from different kukersite seams of the mining area of Aidu opencast mine are presented in
Table 1. The main carbonate mineral is calcite. Dolomite occurs in subordinate
amounts, usually not exceeding 5 %. Close to deep faults, however, dolomite has
often replaced nearly all the calcite [5]. The terrigenous component is
presented by silt-size quartz, feldspar and clay minerals - illite with traces
of chlorite. The content of pyrite is 1-3 %.
Table 1. Main minerals
and kerogen, and their content
in productive seams A-F of kukersit (dry weight)
Mineral
|
Formula |
Wt.% range |
Average |
Mol |
Quartz
|
SiO2 |
3-11 |
4.8 |
0.80 |
|
Orthoclase |
KAlSi3O8 |
1-5 |
3.1 |
0.11 |
|
Illite |
K0.6Mg0.25Al2.3Si3.5O10(OH)2 |
4-17 |
9.1 |
0.24 |
|
Chlorite |
(Mg,Fe2+,Fe3+)6 ·
AlSi3O10(OH)8 |
0-2 |
0.4 |
»0.01 |
|
Pyrite |
FeS2 |
1-3 |
1.6 |
0.13 |
|
Calcite |
CaCO3 |
25-50 |
39.0 |
3.9 |
|
Dolomite |
CaMg(CO3)2 |
0-5 |
1.7 |
0.092 |
|
Kerogen |
(Mol.%) C 39, H 57, O 3.8, N 0.18, S 0.39 |
32-52 |
40.3 |
|
Formation of Technogenic Minerals
Unburnt Waste Rock Heaps
The waste rock contains 3-6 % of kukersite [6],
the remainder being limestone originating from the layers intercalating with
the kukersite seams. The main chemical reaction inside the waste rock heaps is
oxidation of pyrite, followed by the buffering of the acidity by limestone,
precipitation of ferric oxyhydroxide and, in the case of high amount of pyrite
oxidised per kg of available water (>0.008 mol/kg, [7]), also gypsum. The
buffering reaction is fast and no acidification is caused, as the amount of
calcium and magnesium carbonates available exceeds the amount that is needed to
buffer the acidity by about 400-800 times. Eventually, the carbonate content of
kukersite itself exceeds the needed amount 10-20 times. The leachate of the
waste rock heaps is, therefore, slightly alkaline (pH 7.5-8.5), with
Ca2+ and Mg2+
being the main cations, and ![]() the main anions. The
main reactions are:
the main anions. The
main reactions are:
(1) oxidation of pyrite and precipitation of ferric oxyhydroxide:
FeS2 + 3.75O2
+ 3.5H2O __> Fe(OH)3 + 4H+ + 2![]()
(2) buffering of the acidity by carbonates
in limestone (x >0.75 for calcite
and x ~ 0.5 for dolomite):
(CaxMg1-x)CO3
+ 2H+ __> xCa2+ + (1-x)Mg2+ + CO2 + H2O
(3) dissolution of carbonates by CO2:
(CaxMg1-x)CO3
+ CO2 + H2O __> xCa2+ + (1-x)Mg2+
+ 2![]()
(4) precipitation of gypsum:
Ca2+ + ![]() + 2H2O __> CaSO4 · 2H2O
+ 2H2O __> CaSO4 · 2H2O
Burnt Waste Rock Heaps
The main reason why a waste rock heap starts to
burn is spontaneous combustion, caused by too steep slopes, too large height of
the heaps, as well as too big proportion of the oil shale within the waste
rock. The role of the heterogeneities has also to be considered [8]. Although
the average concentration of pyrite is very small in the waste rock (0.05-0.1
%), it might become a significant trigger of the initial temperature rise, if
the shale is concentrated during the disposal by gravitational forces.
Figure 1 presents
schematically various stages in the heap formation. In stage 1, the waste rock
is deposited through dropping from the certain height. The kukersite lumps are
desintegrated much easier than limestone. Therefore, the gravitational
fractionation occurs, with the fraction with largest particle size and
permeability, as well as highest concentration of limestone accumulating in the
foot (base) area (zone 1 in Fig. 1). Zone 2 is the “intermediate” one, and in zone 3, the waste rock with
smallest particle size and therefore largest oil shale content, as well as
lowest permeability, accumulates. In stage 2, the transport road is
continuously extended and the fractionation occurs along the new slope.

Fig. 1. Formation of a waste rock heap containing oil shale and
limestone.
Stage
1: disposal from the transport system; Stage 2: disposal through continuous
extension of the transport system; Point C
– the most probable point for the start of spontaneous combustion
Regarding spontaneous
combustion of the heap, the area C in
Fig. 1 is the most critical one. Firstly, the concentration of fine-grained oil
shale is so large that fastly oxidising fine-grained pyrite may serve as a
trigger for the initial temperature rise, increasing the temperature of the
waste rock to the region where the organic matter starts to oxidise at high
rate. Secondly, the formation of convective air currents is supported by the
high permeability zone below. And thirdly, as the shale is deposited in C first, the incubation period should
end there also first.
During 1960’s and until mid-70’s, seven heaps
including altogether 6 Mt of the waste rock combusted spontaneously and
burned. The average burning time was about ten years and no methods of
extinguishing were successful. The improvements in disposal technology and
better enrichment of the shale have guaranteed that no new heaps have been
burning during 1980’s and 90’s, with the exception of the fires in the old Küttejőu open pit, where the waste containing oil shale has been
ignited several times.
The surface layers of three of
the burnt heaps were sampled and a drill core material of a burnt heap of Sompa mine was analysed. The main
chemical reactions in the surface layer are connected with the decomposition of
carbonates into oxides and, after the cool-down, formation of hydroxides and
again carbonates:
(5) decomposition of carbonates into lime (CaO) and periclase
(MgO):
(CaxMg1-x)CO3
__> xCaO + (1-x)MgO + CO2
(6) hydration of lime and formation of portlandite:
CaO + H2O __> Ca(OH)2
(7) hydration of periclase into brussite:
MgO + H2O __> Mg(OH)2
(8) reaction of portlandite back to calcite:
Ca(OH)2 + CO2
__> CaCO3 + H2O
The lumps of oil shale in the
waste heaps undergo the reactions between carbonate and silicate phases. The
reaction between calcite,
K-feldspar, illite, pyrite and quartz, as well as sulfur from the kerogen,
leads to the formation of technogenic minerals leucite (KAlSi2O6),
diopside (CaMgSi2O8), gehlenite (Ca2Al2SiO7),
wollastonite (CaSiO3), anhydrite (CaSO4), hematite (Fe2O3)
and lime (CaO). All these minerals were detected from the XRD patterns. These
patterns were not used for quantitative determination of the new minerals
because of the uncertainties in the coefficients of the characteristic peak
intensity ratios and poor crystallinity of the minerals. The approximate
concentrations of these minerals, however, can be calculated on the basis of
the approximate molal concentrations of the primary minerals (Tables 1 and 2).
The number of the newly formed phases that were detected (seven) corresponds to
the number of the main chemical compounds available in the system, thus the
analytically determined phases can be taken as “nominal” minerals for recalculations.
Table 2. Calculation of new minerals formed during burning of kukersit
in the surface layer of the waste rock dumps (on the basis of an average
kukersit sample)
|
minerals |
average kukersit sample
(mol/kg) |
Mr |
Wt.% |
||||||
|
|
CaO |
MgO |
Al2O3 |
SiO2 |
K2O |
Fe2O3 |
SO3 |
|
|
|
Leucite KAlSi2O6 |
|
|
|
|
|
|
|
|
|
|
Diopside CaMgSi2O8 |
|
|
|
|
|
|
|
|
|
|
Gehlenite Ca2Al2SiO7 |
|
|
|
|
|
|
|
|
|
|
Wollastonite CaSiO3 |
|
|
|
|
|
|
|
|
|
|
Anhydrite CaSO4 |
|
|
|
|
|
|
|
|
|
|
Hematite
Fe2O3 |
|
|
|
|
|
|
|
|
|
|
Lime CaO |
1.92 |
|
|
|
|
|
|
56 |
24.9 |
After cooling, lime is turned
to portlandite and a carbonate phase (either calcite or aragonite, another
modification of CaCO3), as given by reactions (6)
and (8). Anhydrite
is turned to
gypsum, but ettringite (3CaO · Al2O3
· 3CaSO4 · 31.5H2O) has been also found. The
presence of MgCO3 has not been detected.
In the drill core material
presenting the deeper parts of the burnt heap, some other minerals were
present, including larnite (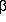 -Ca2SiO4),
tobermorite (4CaO · 5SiO2
· 5H2O),
periclase (MgO) and
spurrite (Ca5Si2O8CO3). In
the samples below 5 m depth in the drill core from the top of a conical heap,
all new minerals contained Ca except periclase. The possible explanation is,
that at higher temperature conditions, the decomposition products of not only
carbonates available in kukersite, but also limestone surrounding kukersite
react with the kukersite lumps that leads to the total domination of Ca and Mg
compounds in the mixture.
-Ca2SiO4),
tobermorite (4CaO · 5SiO2
· 5H2O),
periclase (MgO) and
spurrite (Ca5Si2O8CO3). In
the samples below 5 m depth in the drill core from the top of a conical heap,
all new minerals contained Ca except periclase. The possible explanation is,
that at higher temperature conditions, the decomposition products of not only
carbonates available in kukersite, but also limestone surrounding kukersite
react with the kukersite lumps that leads to the total domination of Ca and Mg
compounds in the mixture.
The "Black Blocks" Zones
On
the southern slope of the Kukruse burnt heap, the local conditions differing
from other parts of the heap and from the other heaps were found. Twelve years
after the burning was considered to be ended, the surface temperature in this
area was still 50 °C, the pores of the surface layer partly saturated
with the shale oil and the vegetation was destroyed. Obviously, the process of
semicoking was still continuing inside the heap. Chesnokov et al. [9] have
described similar zones and formation of graphite layers on the waste rock
lumps in waste rock heaps of Chelyabinsk coal basin, naming these zones as
“black blocks”. In the flow channels of the hot gases onto the surface, the
lumps of limestone were covered with the layer of amorphous carbon (Fig. 2).
Also, cylindrical forms of the same material with the length of up to 10 mm and
diameter of 0.10-0.15 mm were detected.

Fig. 2. Scanning electron micrographs of the amorphous carbon
cover layer consisting of sub-layers
The formation of the
Kukruse heap was analogous to that depicted in Fig. 1, with transport road
approaching from the south. Thus, the area of point C in Fig. 1 matches with the area of “black blocks” of the Kukruse
heap. Possibly, all the oil shale above the point C did not take part in the oxidation process, as the hot gases were
transported out through that material with no oxygen present, leading to the
oil production. Also, at high temperatures, the permeability of the heap slope
could be decreased. In the period of overall cooling of the dump, the process
of semicoking still continues, generating oil and forming the layers of
amorphous carbon.
Environmental Impact of the Burnt Heaps
According
to the hydrochemical analysis using computer code PHREEQC, the mineralogical
composition of the burnt heaps gives a reason to suppose that the leachate
flowing out of the heaps could be highly alkaline (pH up to 12.4) and also with
high sulfate content, as portlandite and gypsum are the most soluble minerals
among these described in this study. However, the temperature in the deeper
layers stays high for a very long time and should be monitored in order to
understand the long term impacts. From the borehole that was drilled 12 years
after the last burning evidence in Sompa, the temperatures up to 240 °C were measured at the
bottom of the hole (depth 23 m). Hence, the infiltrating water either
evaporates or is used up in hydration reactions during decades after burning,
explaining also the formation of tobermorite. Therefore, the period when the
alkaline leachate begins to be produced might be even longer, of the order of a
couple of decades after the end of burning.
The burnt heaps include zones where kerogen of
kukersite is not totally oxidised and the shale oil is still present. This
should be considered when the plans of the use or mass movement of the waste
rock is planned. Also, the movement of oil plumes may contaminate the ground
water resources.
Conclusions
1.
The
main geochemical reaction in the unburnt heaps is oxidation of pyrite, and the
only new solid phases detected were ferric oxy-hydroxide and gypsum. In the
samples from the burnt heaps, a large number of technogenic minerals were
found, including lime, periclase, portlandite, brussite, calcite, aragonite,
leucite, diopside, gehlenite, wollastonite, anhydrite, ettringite, hematite,
tobermorite, larnite and spurrite.
2.
The
geochemical calculations made on the molal basis of the unchanged and burnt
kukersite indicate that the analytically determined minerals can be used as the
“nominal” minerals describing the character of change.
3.
The
phenomenon of amorphous carbon formation in the “black blocks” zone together
with the abundance of oil in the surface layer are evidences that not all of
the kukersite is fully oxidised. The formation of the “black blocks” in certain
areas can be explained by the gravitational fractionation of the waste rock
material during disposal.
4.
The
negative environmental impact of the burnt heaps may become evident after
decades after burning only, because the temperature inside the heaps decreases
very slowly and practically no leachate is formed. The potential problems are
connected with high alkalinity, sulfate content, as well as with migration of
oil plumes.
Acknowledgements
The assistance of Prof. Enno Reinsalu and Dr.
Tarmo Kiipli during the first field work period in 1988 is greatfully
acknowledged. The comments of a reviewer, Dr. Rein Kuusik were appreciated.
References
1. Utsal, K. Comprehensive investigation of oil shale material
composition by X-ray diffrection method // Oil Shale. 1984. Vol. 1, No. 1. P.
69-80.
2. Parkhurst,
D. L. Users guide to PHREEQC – A computer
program for speciation, reaction-path, advective-transport, and inverse
geochemical calculations // USGS Water Resources Investigations Report 95-4227.
Lakewood, Colorado, 1995. P. 143.
3. Ball, J.
W., Nordstrom, D. K. WATEQ4F – User’s manual with
revised thermodynamic data base and test cases for calculating speciation of
major, trace and redox elements in natural waters // USGS Open-File Report
90-129. 1991. P. 185.
4.
Puura,
V., Bauert, H., Männil, R.
The conditions of kukersite deposition // Proceedings of International
Conference on Oil Shale and Shale Oil (ed. Zhu Yajie). Bejing, China, 1988. P.
42-50.
5. Bauert, H.,
Puura, V. Geology of the Baltic Oil
Shale Basin // Field Meeting Estonia 1990 : An Excursion Guidebook (eds. D.
Kaljo and H. Nestor) / Estonian Academy of Sciences. Tallinn, 1990. P. 40-45.
6. Voolma, E.,
Petersell, V., Güsson, Ü. Development of the plan for the extent of geological
investigations of building materials. 3/1. A study of the solid waste of
enrichment factories, low-quality sands and the profitability of mining granite
// Report of the Tallinn division
of the Geological Survey of ESSR. 1981 [in Russian].
7. Puura, E. Weathering of mining waste rock containing alum shale
and limestone : A case-study of the Maardu dumps, Estonia; PhD thesis /
Department of Chemical Engineering and Technology, Royal Institute of
Technology. Stockholm, 1998. P. 136.
8. Puura, E.,
Pihlak, A. Oxidation of Dictyonema shale
in Maardu mining waste dumps // Oil Shale. 1998. Vol. 15, No. 3. P. 239-267.
9.
Chesnokov,
B. V., Bazhenova, L. F., Shcherbakova, E. M., Mihal, T. A., Deryabina, T. N.
Mineralogy of the burnt dumps of the Chelyabinsk coal basin
/ Academy of Sciences of the USSR, Ural Science Centre. - Sverdlovsk, 1987. P.
70 [in Russian].